Detailed product information can be found here
QUALITATIVE AND QUANTITATIVE COMPOSITION
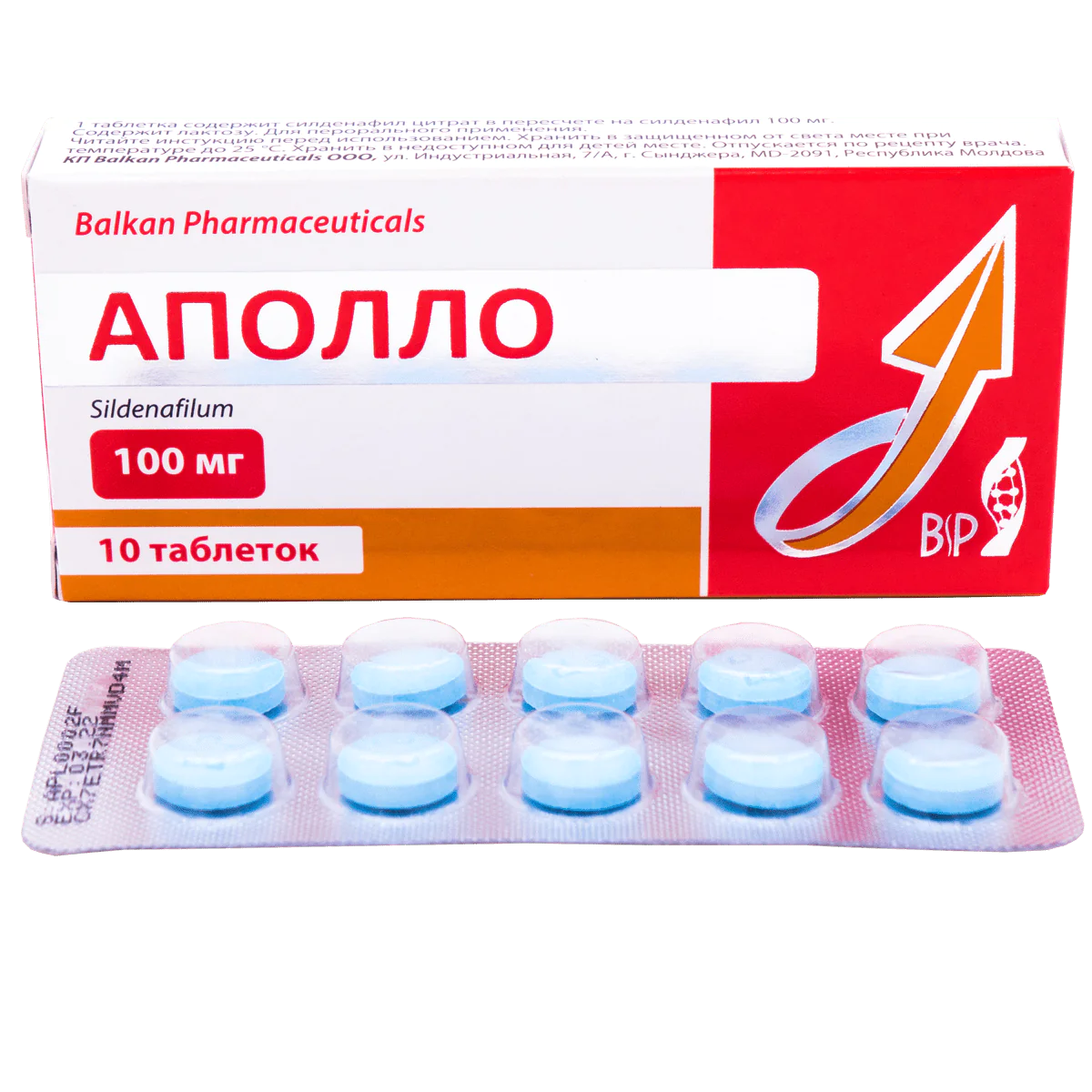
BP Apollo 100 (Sidenafil) mg tablets
Each tablet contains sildenafil citrate, equivalent to 100 mg sildenafil. Excipient with known effect: Each tablet contains 122 mg of lactose (as monohydrate).
PHARMACEUTICAL FORM Tablets.
APOLLO 100 mg tablets
Round, biconvex blue tablets with “BP” in one side. Marble is allowed on the surface of the tablets.
CLINICAL DATA
Therapeutic indications
APOLLO is indicated for adult men with erectile dysfunction who are unable to achieve or maintain a penile erection sufficient for satisfactory sexual intercourse.
For APOLLO to be effective, sexual stimulation is required.
Posology and mode of administration
Dosage
Administration to adults
The recommended dose is 50 mg sildenafil administered approximately one hour before sexual activity. Depending on efficacy and tolerability, the dose may be increased to 100 mg sildenafil, or may be lowered to 25 mg sildenafil. The maximum recommended dose is 100 mg sildenafil. The maximum recommended intake frequency is once a day. If APOLLO is taken during the meal, the effect is slower than when given as fast-food .
Special patient groups
Elderly patients
Patients with renal impairment
In patients with mild to moderate renal impairment (creatinine clearance = 30-80 ml / min), the same doses as those listed in the section “Adult Administration” are recommended.
Since clearance of sildenafil is low in patients with severe renal impairment (creatinine clearance <30 ml / min), a 25 mg dose should be considered. Depending on efficacy and tolerability, the dose may be increased gradually to 50 mg or 100 mg, if necessary.
Patients with hepatic impairment
Because sildenafil clearance is low in patients with hepatic impairment (for example cirrhosis), an initial dose of 25 mg sildenafil should be considered. Depending on efficacy and tolerability, the dose may be increased gradually to 50 mg sildenafil and up to 100 mg sildenafil, if necessary.
Children and adolescents
APOLLO is not indicated in people under the age of 18 years.
Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1. Consistent with the known effects of sildenafil on nitric oxide / cyclic guanosine monophosphate (GMPc) (see section 5.1), it has been shown to potentiate the hypotensive effects of nitrates and therefore concomitant administration of sildenafil with nitric oxide donors ( such as amyl nitrite) or any form of nitrate.
Medicines for the treatment of erectile dysfunction, including sildenafil, should not be administered to men in whom sexual activity is not indicated (for example patients with severe cardiovascular disorders such as unstable angina or severe heart failure).
APOLLO is contraindicated in patients with ocular blindness due to non-arterial ischemic anterior neuropathy (NOAIN), whether or not this episode correlated with previous exposure to PDE5 inhibitors (see section 4.4).
The safety of sildenafil has not been studied in the following subgroups of patients and therefore its use is contraindicated in these cases: severe hepatic impairment, hypotension (blood pressure <90/50 mmHg), recent history of stroke or myocardial infarction, known retinal degenerative hereditary diseases such as pigmentary retinas (a small proportion of these patients present genetic disorders of retinal phosphodiesterases).
Special warnings and precautions for use
Before recommending a pharmacological treatment for erectile dysfunction, personal history and physical examination should be assessed to diagnose erectile dysfunction and establish potential preexisting etiologies.
Cardiovascular risk factors
Before initiating any treatment for erectile dysfunction, physicians should assess the cardiovascular status of patients as there is a degree of cardiac risk associated with sexual activity. Sildenafil has vasodilating properties, resulting in a mild and transient decrease in blood pressure (see section 5.1). Before prescribing sildenafil, physicians should carefully investigate how patients with certain pre-existing conditions may be affected by these vasodilatory effects, especially in association with sexual activity. In the category of patients with increased susceptibility to vasodilatory medicinal products, patients with left ventricular ejection pathway obstruction (for example aortic stenosis, obstructive hypertrophic cardiomyopathy) or those with rare systemic multiple atrophy manifested by severe impairment of autonomic tension control pressure.
Most, but not all, of these patients had pre-existing cardiovascular risk factors. Many events have been reported to occur during or shortly after sexual intercourse, some of which have been reported to occur shortly after sildenafil administration and without sexual intercourse. It is impossible to determine whether these events are directly related to these factors or whether they are determined by other factors.
Priapism
Medicines indicated for the treatment of erectile dysfunction, including sildenafil, should be used with caution in patients with anatomical penile deformities (such as angulation, cavernosal fibrosis or Peyronie’s disease) or those with pruritus predisposing conditions (such as sickle cell disease, multiple myeloma or leukemia).
In the post-marketing experience of sildenafil, prolonged erections and priapism have been reported. If an erection persists for more than 4 hours, the patient should seek urgent medical attention. If priapism is not treated immediately, there is a risk of penile tissue damage and permanent impotence.
PHARMACOLOGICAL PROPERTIES
Pharmacodynamic properties
Pharmacotherapeutic group: Drugs for the treatment of erectile dysfunction.
Mechanism of action
Sildenafil is an orally administered drug for the treatment of erectile dysfunction. Under natural conditions, that is, in the presence of sexual stimulation, sildenafil restores affected erectile function by increasing blood flow to the penis.
The physiological mechanism responsible for penile erection involves the release of nitric oxide (NO) in the cavernous body during sexual stimulation. Nitric oxide activates the guanylate-cyclase enzyme, which increases the levels of cyclic guanosine monophosphate (GMPc), producing relaxation of the smooth muscles of the cavernous body and thus favoring blood flow.
Sildenafil is a potent and selective PDE5-specific GMPc phosphodiesterase (PDE5), which is responsible for the degradation of GMPc in the cavernous body. Sildenafil influences erection through its peripheral action. Sildenafil does not have a direct relaxing effect on the isolated human tissue from the cavernous body but strongly increases the NO’s relaxing effect on this tissue. When activating the NO / GMPc pathway by sexual stimulation, inhibition of PDE5 by sildenafil induces increased GMPc concentrations in the cavernous body. Therefore, sexual stimulation is required for sildenafil to produce its desired pharmacological effect.
Pharmacodynamic effects
In vitro studies have shown that sildenafil has selectivity for PDE5, which is involved in the erection process. Its effect is stronger on PDE5 than on other known phosphodiesterases. There is a 10-fold selectivity for PDE5 than for PDE6 involved in retinal phototransduction. At the maximum recommended doses, selectivity is greater than 80 times the selectivity for PDE1 and over 700 times the selectivity for PDE2, 3, 4, 7, 8, 9, 10 and 11. In particular, sildenafil has a selectivity of 4000 fold greater for PDE5 than for PDE3, which is the isoform of phosphodiesterase specific for AMPc involved in regulating cardiac contractility.
Clinical efficacy and safety
Two clinical trials have been specifically designed to evaluate the time interval between sildenafil dosing and the occurrence of erection in response to sexual stimulation. In the study in which sildenafil was given under fasting conditions, penile plethysmography (RigiScan) showed that the average time to achieve an erection with 60% stiffness (sufficient for sexual intercourse) was 25 minutes (with an interval of 12 – 37 minutes). In a separate study using RigiScan, it was observed that an erection was obtained 4-5 hours after sildenafil administration in response to sexual stimulation. Sildenafil produces mild and transient decreases in blood pressure, which in most cases do not produce clinical effects. After an oral 100 mg dose of sildenafil, the mean maximum reduction in systolic blood pressure at baseline was 8.4 mmHg. The corresponding reduction in diastolic blood pressure was 5.5 mmHg. These blood pressure reductions are consistent with the vasodilator effects of sildenafil, probably due to increased concentrations of GMPc in the vascular smooth muscle.
In healthy volunteers, single oral doses of sildenafil up to 100 mg did not cause any significant effect on the ECG.
In a study of the hemodynamic effects of oral administration of a single 100 mg sildenafil dose in 14 patients with severe coronary disease (stenosis> 70% on at least one artery coronary artery disease), mean values of systolic and diastolic blood pressure at baseline decreased by 7% and 6% relative to baseline. Systolic mean pulmonary blood pressure decreased by 9%. It has been shown that sildenafil does not influence cardiac output or blood flow in stenotic coronary arteries.
In a double-blind, placebo-controlled trial of exercise tolerance, 144 patients with erectile dysfunction and stable chronic angina, treated with anti-anginal drugs (without nitrates), were evaluated. The results did not indicate any clinically significant difference between the sildenafil and placebo group in terms of time to the occurrence of limbic angina attacks.
In some subjects examined on the Farnsworth-Munsell test on 100 shades of color, 1 hour after administration of a 100 mg dose of sildenafil, slight and transient differences in blue / green color perception were observed, and after No obvious effect was observed 2 hours after dosing. The postulated mechanism for this change in color perception mode is correlated with PDE6 inhibition involved in the retinal phototransduction cascade. Sildenafil has no effect on visual acuity or on visual contrast sensitivity.
In a placebo-controlled clinical trial in a small number of patients diagnosed with age-related macular degeneration (n = 9), sildenafil (single dose of 100 mg) did not cause significant visual impairment (visual acuity , Amsler grid, traffic light simulation for differential color perception, Humphrey perimeter and photostresis).
In healthy volunteers, no changes in sperm motility or morphology were observed after oral administration of a single dose of sildenafil 100 mg (see section 4.6).
Additional information from clinical trials
In clinical trials, sildenafil was administered to over 8000 patients aged 19-87 years, in the following categories: the elderly (19.9%), patients with hypertension (30.9%), diabetic patients ( 20.3%), ischemic heart disease (5.8%), hyperlipidemias (19.8%), spinal cord injuries (0.6%), depression (5.2%), transurethral prostate resection (3, 7%), radical prostatectomy (3.3%). The following categories of patients have not been well studied or excluded from clinical trials: patients with pelvic surgery, radiotherapy, patients with severe renal impairment or severe hepatic impairment, and patients with certain cardiovascular disorders (see section 4.3).
In fixed dose studies, the percentages of patients who reported improved erection due to treatment were 62% at the 25 mg dose, 74% at the 50 mg dose and 82% at the 100 mg dose versus 25% in the in case of placebo. In controlled clinical trials, the frequency of withdrawal from sildenafil due to adverse reactions was small and comparable to that seen with placebo. In all clinical trials, the percentage of patients who reported improvement from sildenafil treatment was 84% (in patients with psychotic erectile dysfunction), 77% (in mixed erectile dysfunction), 68% (in patients with erectile dysfunction), 67% (in the elderly), 59% (in diabetic patients), 69% (in patients with myocardial ischaemia), 68% (in patients with hypertension), 61% (in patients with resection prostate transurethral), 43% (in patients with radical prostatectomy), 83% (in patients with spinal cord injuries), 75% (in patients with depression). Long-term studies have shown to maintain the safety and efficacy of sildenafil treatment.
Children and adolescents
The European Medicines Agency has waived the obligation to submit the results of studies with the reference medicine containing sildenafil in all subgroups of pediatric patients for the treatment of erectile dysfunction. See section 4.2 for information on use in children and adolescents.
5.2 Pharmacokinetic properties
Absorption:
Sildenafil is rapidly absorbed. After oral dosing, in fasting conditions, peak plasma concentrations are reached in 30 – 120 minutes (with an average of 60 minutes). The mean oral bioavailability is 41% (between 25-63%). Following oral administration, sildenafil AUC and Cmax, increase in dose proportionality within the recommended dose range (25-100 mg).
If sildenafil is administered with food, the rate of absorption decreases with an average delay of 60 minutes of Tmax, and an average reduction of 29% of Cmax.
Distribution:
The mean steady-state volume distribution (Vd) for sildenafil is 105 l, indicating the distribution in the tissues. Following oral single dose of 100 mg, the mean maximum sildenafil peak plasma concentration is approximately 440 ng / ml (40% VC). Since sildenafil (and its main circulating metabolite, N-desmethyl) binds to 96% of plasma proteins, an average mean peak plasma sildenafil concentration of 18 ng / ml (38 nM) results. Plasma protein binding is independent of the total plasma concentration of the drug.
In healthy volunteers using sildenafil (100 mg single dose), less than 0.0002% (mean 188 ng) of the administered dose is present in the ejaculate after 90 minutes of dosing.
Metabolism:
Sildenafil is primarily metabolised by the CYP3A4 (main pathway) and CYP2C9 (secondary pathway) microsomal enzymes. N-demethylation of sildenafil results in the main circulating metabolite. This metabolite has a selectivity profile for PDE similar to sildenafil and a PDE5 inhibitory potency in vitro of about 50% of that of the non-metabolised drug. Plasma concentrations of this metabolite are approximately 40% of those of sildenafil. The N-demethyl metabolite is further metabolised with an elimination half-life of approximately 4 hours.
Elimination:
The total body clearance of sildenafil is 41 l / h, resulting in a half-life of elimination half-life of 3-5 hours. After oral or intravenous administration, sildenafil is excreted as metabolites, predominantly by feces (approximately 80% of the oral dose) and less urine (approximately 13% of the oral dose).
Pharmacokinetics in special patient groups
Elderly
Healthy elderly volunteers (65 years of age or older) experienced a decreased clearance of sildenafil, with approximately 90% increases in plasma levels of sildenafil and the active N-demethyl metabolite compared to those observed in young healthy volunteers (18-45 years). Due to the different proportions of age-related plasma protein binding, the increase in free sildenafil plasma concentrations was approximately 40%.
Kidney failure
Following oral administration of a single dose of 50 mg of sildenafil to volunteers with mild to moderate renal impairment (creatinine clearance = 30-80 ml / min), the pharmacokinetics of sildenafil have not been altered. The mean AUC and Cmax of the N-demethyl metabolite increased by 126% and 73%, respectively, compared to those obtained in volunteers of the same age without any renal dysfunction. However, taking into account the large variability among subjects, these differences did not show statistical significance. In volunteers with severe renal impairment (creatinine clearance <30 ml / min), sildenafil clearance was low, with a mean AUC increase of 100% and Cmax by 88% compared to those of the same age group without impairment. In addition, the AUC and Cmax of the N-desmethyl metabolite were significantly increased by 79% and 200%, respectively.
Hepatic impairment
In patients with mild to moderate hepatic cirrhosis (Child-Pugh A and B) clearance of sildenafil was low, resulting in increases in AUC (84%) and Cmax (47%) compared with volunteers of the same age and without hepatic impairment. In patients with severe hepatic impairment, the pharmacokinetics of sildenafil have not been studied.
5.3 Preclinical safety data
Animal studies have not demonstrated the existence of a specific human hazard based on conventional studies of safety pharmacology, repeated dose administration, genotoxicity, carcinogenicity, reproductive toxicity and developmental toxicity.
- PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Microcrystalline cellulose
Lactose monohydrate
Croscarmellose sodium
Kollidon 25 (polyvinylpyrrolodone)
Magnesium stearate
Color FDC Blue N.1.
6.2 Incompatibilities
It’s not necessary.
6.3 Shelf life
36 months.
6.4 Special precautions for storage
Keep protected from light and temperatures below 25 ° C.
Keep out of the reach and sight of children.
6.5 Nature and contents of container
APOLLO 50 mg tablets
The tablets are packaged in PVC/Al blisters. The secondary pack is a carton box with 1 blister of 2, 5 or 20 tablets.
APOLLO 100 mg tablets
The tablets are packaged in PVC/Al blisters. The secondary pack is a cardboard box with 1 blister of 2, 4 or 10 tablets.
6.6 Special precautions for disposal and other handling
No special requirements.
- CERTIFICATE OF REGISTRATION
SC Balkan Pharmaceuticals SRL N. Grădescu str., 4, MD-2002, or. Chisinau, Republic of Moldova.
The manufacturer
SC Balkan Pharmaceuticals SRL Industrial Street, 7 / A, MD-2091, or. Singera, Republic of Moldova.




Archer Bailey –
Apollo works well, I advise you to use it.
It is better to drink on a slightly empty stomach, I do not recommend it after meals. The head may hurt, and the pressure rises, the eyes are watery, but all this is individual.
Etan Bowlix –
I think it will suit any man who is over 40 or has gone too far with steroids. This drug helps me perfectly. I bought it and never regretted it, the price is not as high as that of Viagra in the pharmacy, and there are no side effects from it.