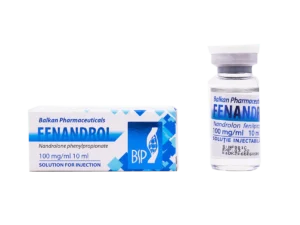
Package leaflet: information for the user / patient fenandrol 100 mg / ml solution for injection
Nandrolone phenylpropionate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist or health care provider.
• This medicine has been prescribed for you only. You do not have to give it to others. It can harm them, even if they have the same signs of illness as yours.
• If you have any side effects, talk to your doctor or pharmacist or nurse. These include any possible side effects not mentioned in this leaflet. See section 4.
What you find in this leaflet:
1. What Fenandrol is and what it is used for
2. What you need to know before you use Fenandrol
3. How to use Fenandrol
4. Possible side effects
5. How to store Fenandrol
6. Packaging content and other information
1. What Fenandrol is and what it is used for
Fenandrol contains nandrolone phenylpropionate as active substance in the group of anabolic steroids.
Fenandrol is indicated for the treatment of anemia as a result of chronic renal failure during convalescence after severe trauma, infectious diseases, burns, surgery, radiation therapy.
If you have any questions about Fenandrol’s directions and how it works, talk to your doctor or pharmacist.
2. What you need to know before you use Fenandrol
Do not take Fenandrol:
• if you are allergic (hypersensitive) to the active substance or any of the other ingredients of the preparation;
• if you have prostate cancer, breast cancer in men, breast cancer in women with hypercalcaemia, ischemic heart disease, severe atherosclerosis, nephrotic syndrome, acute and chronic liver disease including alcohol, nephritis;
• pregnancy, lactation period;
• it is not recommended for children and adolescents under the age of 18 due to a lack of data on safety and efficacy.
Tell your doctor or pharmacist if you are in one of the above cases, because in these situations Fenandrol is not suitable for you.
Take special care with Fenandrol
It is forbidden to overdose recommended doses.
When the first signs of virilization occur in women (voice thickening, hirsutism, acne, clitoromalgia), administration should be stopped to avoid irreversible changes. Systemically control lipidemia and cholesterol (the level of fat in the blood). When administering the preparation to women, the expected benefit and the possible risk of treatment should be evaluated considering the androgenic effect of the preparation.
Treatment with Fenandrol requires caution in (or a history of) heart disease and circulatory disorders, liver and kidney dysfunction, as well as epilepsy, migraine and glaucoma. During treatment with Fenandrol, it is recommended to check the intraocular pressure (for example eye pressure) regularly that there is a chance of retention of sodium and water. Hepatic impairment may occur, hepatic function testing should therefore be performed after 4 weeks of treatment. Before initiating the treatment and later on, prostate rectal control is recommended during the treatment. In diabetes, it is advisable to adjust the doses of anti-hyperglycaemic agents.
In patients with malignant tumors the duration of treatment will be determined individually and according to liver parameters.
It is given to athletes only for the purpose of treating the recommended pathologies.
Fenandrol may influence anti-doping tests in athletes.
Tell your doctor or pharmacist if you are in one of the above cases.
Taking other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription but especially medicines: glucocorticoids, mineralocorticoids, corticotropin, anti-coagulants, insulin and oral antidiabetics, somatotropin and its derivatives.
Using Fenandrol with food and drink
Sodium-rich foods potentiate fluid retention in the body, increase the risk of developing edema, intensify acne eruptions.
Pregnancy and breast-feeding
Fenandrol is contraindicated during pregnancy and during lactation.
Driving and using machines
Fenandrol does not affect the ability to drive or operate machinery.
Important information about some components of Fenandrol
Fenandrol contains benzyl alcohol. It should not be given to premature or newborn infants.
3. How to use Fenandrol
Always take Fenandrol as your doctor has told you.
You should talk to your doctor or pharmacist if you are not sure.
The drug is administered intramuscularly deeply.
Treatment of anemia as a result of chronic renal insufficiency Intramuscularly deep 100 mg (for women) and 200 mg (for men) once a week. The treatment cure is 4-8 weeks.
If the resulting course is ineffective, treatment must be interrupted.
Convulsion period
Intramuscularly deeply 50 mg once every 3-4 weeks.
If you take more Fenandrol than you should
Cases of overdose have not been reported. If you have been given more Fenandrol, talk to your doctor, pharmacist immediately the nearest emergency receiving units. In the case of abuse of preparations containing nandrolone (administration of high doses as anabolic remedy), severe endocrine, metabolic and psychiatric adverse reactions may occur.
If you forget to take Fenandrol
Take a dose as soon as you remember, then continue on a regular basis only if the time is not very close to the next dose. Do not take a double dose to make up for a forgotten dose.
4. Possible side effects
Like all medicines, Fenandrol can cause side effects, although not everybody gets them.
Stop taking Fenandrol and contact your doctor immediately, if you have any of the following symptoms that may be signs of an allergic reaction:
• difficulty in breathing or swallowing;
• swelling of the face, lips, tongue or throat;
• severe itching, with redness or pustules.
Fenandrol injection in therapeutic doses is unlikely to cause side effects.
During the prolonged treatment, the following side effects (no frequency data available) were observed:
Liver tumors.
Hypersensitivity reactions, inhibition of sex hormone secretion.
Changes in general blood count, hypocoagulation with haemorrhage tendency.
In women, signs of virilization, for example, acne (facial red coats), hirsutism, male alopecia, irreversible thickening of the voice, dysmenorrhea, clitoral hypertrophy. Craving can be the first sign of tonality change, which can be long lasting and sometimes irreversible. Men may develop gynecomastia (swelling of the mammary gland) and inhibition of sperm production.
Adolescent boys can see frequent erections and penis enlargement. Post-puberty may develop gynecomastia, idiopathic cutaneous pigmentation, retention or stopping the growth (calcification of epiphyseal growth zones of tubular bones).
In girls, the increase in pubic hair and clitoral hypertrophy. Change libido.
Fluid retention with peripheral edema, increased blood calcium. Progression of atherosclerosis (fat deposition on the vascular wall). Nausea, vomiting, loss of appetite. Dysplasia of liver function with jaundice (yellowing of the skin) and change (positive) of liver function indices.
Acne, insignificant hirsutism.
Pain in long tubular bones.
Priapism (long erections).
In the post-puberty period – bladder irritation.
Increase in urea concentration (biochemical index) in blood calcium.
Reporting Adverse Reactions
If you experience any side effects, talk to your doctor or pharmacist. These include any possible side effects not mentioned in this leaflet. By reporting side effects, you can help provide additional information on the safety of this medicine.
5. How to store Fenandrol
Keep out of the reach and sight of children.
Keep protected from light and temperatures below 25 °C.
Do not use Fenandrol after the expiry date, which is stated on the pack after “EXP”. The expiration date refers to the last day of that month.
No special storage conditions are required.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines you no longer need. These measures will help to protect the environment.
6. Packaging content and other information
What Fenandrol contains 100 mg/ml solution for injection
The active substance is nandrolone phenylpropionate.
Each 1 ml of solution for injection contains 100 mg of nandrolone phenylpropionate.
Each 1 ml ampoule contains 100 mg nandrolone phenylpropionate.
Each 10 ml vial contains 1000 mg nandrolone phenylpropionate.
The other ingredients are: benzyl alcohol, benzylbenzoate, peach oil.
What Fenandrol looks like and contents of the pack
Clear, transparent, colorless or light yellow solution. Cardboard box with 1 ml ampoules of two (2) PVC holders each containing five (5) ampoules.
Cardboard box with 1 vial of 10 ml.
CERTIFICATE OF REGISTRATION
SC Balkan Pharmaceuticals SRL N. Grădescu str., 4, MD-2002, or. Chisinau, Republic of Moldova.
The manufacturer
SC Balkan Pharmaceuticals SRL Industrial Street, 7/A, MD-2091, or. Singera, Republic of Moldova.
This product you can buy here.