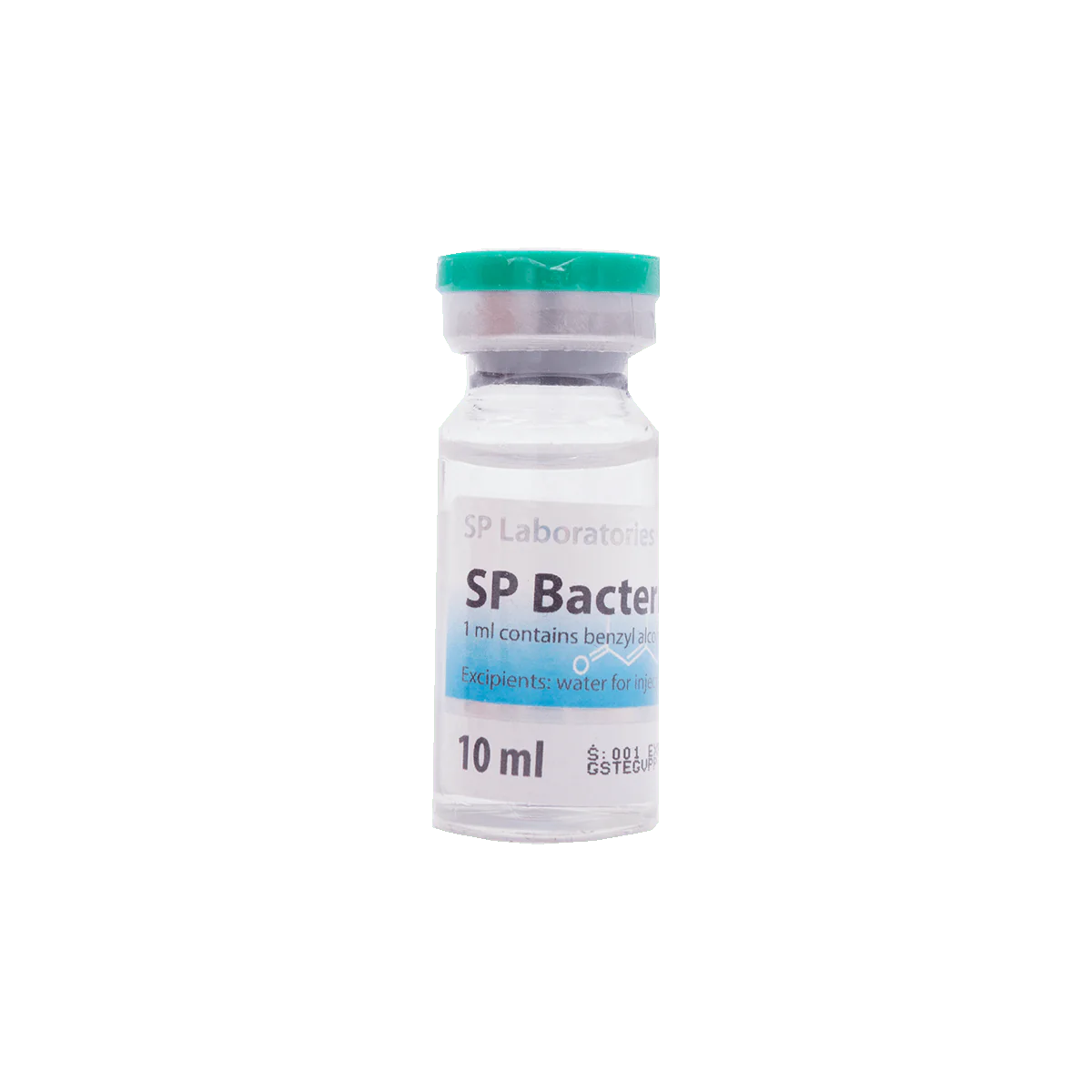
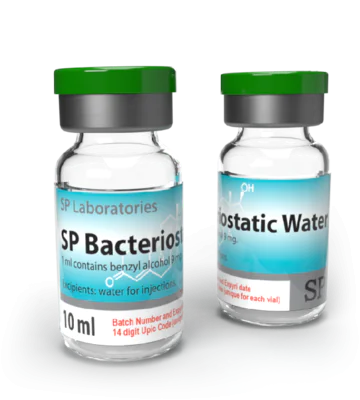
Bacteriostatic Water (bacteriostatic water for injection) is sterile water containing 0.9% benzyl alcohol that is used to dilute or dissolve medications; the container can be reentered multiple times (usually by a sterile needle) and the benzyl alcohol suppresses or stops the growth of most potentially contaminating bacteria. Bacteriostatic Water is available generically and has the same name. The Bacteriostatic Water can be used in diluting drugs that can subsequently be administered by intravenous, intramuscular, or subcutaneous injection. There are few side effects of Bacteriostatic Water, if any, in adults and if they occur, it they may be related to contamination, particulate matter or the drug that is being dissolved (see below). Side effects that may occur after drugs are added to Bacteriostatic Water include fever, abscess formation, venous thrombosis or phlebitis, tissue death, and infections.
Bacteriostatic Water for Injection
Is supplied in a multiple-dose 10 mL glass vial that is pressurized. It is not used for neonatal medications because of possible blood pressure changes and toxicity of benzyl alcohol. If Bacteriostatic Water is injected intravenously without any diluted compound, it may cause some red blood cell lysis because it is not isotonic. Bacteriostatic Water usually does not interact with other drugs; however, some drugs for injection may be incompatible. Tell your doctor all medications and supplements you use. Tell your doctor if you are pregnant before receiving Bacteriostatic Water. Consult your doctor before breastfeeding.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects.
SIDE EFFECTS
Reactions which may occur because of this solution, added drugs or the technique of reconstitution or administration include febrile response, local tenderness, abscess, tissue necrosis or infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection and extravasation.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate countermeasures, and if possible, retrieve and save the remainder of the unused vehicle for examination.
Although adverse reactions to intravenous, intramuscular or subcutaneous injection of 0.9% benzyl alcohol are not known to occur in man, experimental studies of small volume parenteral preparations containing 0.9% benzyl alcohol in several species of animals have indicated that an estimated intravenous dose up to 30 mL may be safely given to an adult without toxic effects.
Administration of an estimated 9 ml to a 6 kg infant or neonate is potentially capable of producing blood pressure changes.






Jackson –
Comfortable, stylish, really works